One of the more common genetic heart conditions we confront with patients is one known as hypertrophic cardiomyopathy (HCM). I am pleased to say that in many cases, we can improve symptoms considerable with medications but in some individuals, we need to consider additional interventions to help control symptoms and therefore improve quality of life. These interventions are frequently invasive including open heart surgery. Therefore, the recent inclusion of a new medicine called Mavacamten has been greeted with significant excitement for patients with this condition. So, let’s discuss briefly what HCM is and also evaluate some recent promising results with Mavacamten in the management of HCM.
Understanding Hypertrophic Cardiomyopathy (HCM)
Hypertrophic cardiomyopathy (HCM) is a complex and often misunderstood heart condition that affects the structure of the heart muscle. It’s a type of cardiomyopathy, which refers to diseases that affect the heart’s ability to pump blood effectively. HCM is characterized by an abnormal thickening of the heart muscle, particularly the left ventricle—the chamber responsible for pumping oxygen-rich blood out to the body.
This thickening, known as hypertrophy, can make the heart muscle stiff and less able to relax between beats, hindering the heart’s pumping efficiency. This condition is hereditary and can develop at any age. While many individuals with HCM experience no symptoms or only mild ones, it can lead to serious complications, including arrhythmias (abnormal heart rhythms), heart failure, and even sudden cardiac arrest.
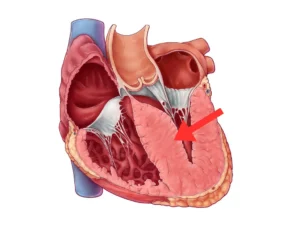

Genetic mutations are a key factor in the development of HCM. These mutations affect proteins in the heart muscle cells, leading to the characteristic thickening and disarray of the muscle fibers. The symptoms of HCM can vary widely and may include chest pain, shortness of breath, fatigue, dizziness, fainting, and heart palpitations. These symptoms often arise due to the heart’s struggle to pump blood effectively or irregular electrical signals within the heart.
Diagnosis of HCM involves a comprehensive assessment, including a physical exam, family history evaluation, echocardiogram, and sometimes additional imaging tests like cardiac MRI. An electrocardiogram (ECG) and stress test may also help in evaluating the heart’s electrical activity and response to exertion. Genetic testing can identify mutations linked to HCM, aiding in both diagnosis and assessing the risk of sudden cardiac events among family members.
Managing HCM typically involves a multi-faceted approach. Medications may be prescribed to alleviate symptoms and reduce the risk of complications. Lifestyle modifications, including avoiding intense physical exertion, managing stress, and maintaining a heart-healthy diet, are important. For individuals at higher risk, implantable devices like pacemakers or defibrillators might be recommended. In some cases, surgical procedures or alcohol septal ablation may be considered to alleviate obstruction of blood flow within the heart.
How Mavacamten works
Mevacampten targets the cardiac muscle itself and is a small molecule inhibitor of the cardiac myosin protein, which plays a crucial role in the contraction of the heart muscle. By inhibiting the myosin protein, Mevacampten reduces the force and velocity of the heart muscle contraction, which can alleviate the symptoms and complications of HCM. In addition, Mevacampten has shown a favorable safety and tolerability profile in clinical trials, with no serious adverse events reported [1].
What are the benefits of Mavacamten?
Mavacamten has been proven effective in reducing the left ventricular outflow tract gradient (LVOTG), a common characteristic of HCM that contributes to symptoms such as chest pain, shortness of breath, and dizziness. The outflow tract refers to the section of the left ventricle that pumps blood out of the heart and into the aorta, delivering vital nutrients and oxygen to the body. Increased ventricular pressures can cause many of the symptoms associated with HCM, including shortness of breath, fluid buildup, chest pain, dizziness, and fainting. For some patients, these symptoms can significantly affect their quality of life, making any treatment that can alleviate them a welcome development.
Several clinical trials have investigated the efficacy and safety of Mavacamten in patients with HCM. One of the most recent studies, published in the Lancet in 2020, enrolled 251 patients with symptomatic obstructive HCM and randomized them to receive either Mavacamten or placebo for 30 weeks. The results showed that Mavacamten significantly reduced the pressure within the left ventricle (left ventricular outflow tract gradient) and improved symptoms such as dyspnea and exercise capacity compared to placebo, with no significant adverse effects [1]. In detail, by the end of the study, 37% of participants treated with Mavacamten improved on an endpoint measuring exercise capacity and symptoms, compared to 17% of participants in the placebo group [1].
Given the benefits of this medicine, the FDA have approved this for use on symptomatic patients. It has also received approval in other countries including recently in Australia by the Therapeutics Goods Administration.
Dosing and Administration
Mavacamten (trade name: Camzyos) is taken once daily and comes in capsule form with dosage varying between 2.5mg to 15mg. The dosing of Mavacamten can vary depending on individual factors such as your medical condition, age, weight, and response to the treatment. It’s essential to follow the dosing instructions provided by your healthcare provider closely. Typically, the initial dosing regimen involves gradually increasing the dose to reach the target dose over a few weeks. Your doctor will monitor your response and adjust the dosage as needed to achieve the best results.
Safety Monitoring when using Mavacamten
Mavacamten can cause heart failure by reducing heart muscle contraction. Patients with intercurrent illnesses or arrhythmias are at higher risk of developing this condition when using the medication. Close monitoring of the medication’s dosage and symptoms, along with echocardiogram findings (heart ultrasound that is useful and accurate in picking up how the heart is pumping), is necessary to detect any changes requiring dose adjustment or cessation. Certain medications can interfere with Mavacamten’s metabolism, so patients must consult their doctor and pharmacist before starting treatment.
Conclusion
In conclusion, Mavacamten represents a novel and promising option for the management of HCM, particularly in patients with symptomatic obstructive disease who are not adequately controlled with current therapies. However, as with any new medication, the use of Mavacamten should be carefully considered in each individual case based on the patient’s clinical condition, medical history, and preferences, in consultation with a qualified healthcare provider.
Reference:
- Olivotto, I., Oreziak, A., Barriales-Villa, R., Abraham, T. P., Masri, A., Garcia-Pavia, P., Saberi, S., Lakdawala, N. K., Wheeler, M. T., Owens, A., Salerno, J. C., Poillucci, G., Rapezzi, C., Hong, S. N., Maron, B. J., Rowin, E. J., Jain, A., Sehnert, A. J., Green, E. M., & Ho, C. Y. (2020). Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 396(10253), 759-769. doi: 10.1016/S0140-6736(20)31859-1.