Vitamin D is a fat-soluble vitamin that plays a crucial role in the body’s calcium absorption, essential for bone health. However, recent research has suggested that vitamin D may also be important for heart health. In this article, we’ll examine the relationship between vitamin D3, K2, and heart health. We’ll discuss the mechanisms behind their protective effects, investigate the latest scientific findings, and explore practical strategies for optimizing their intake. Whether you’re seeking to enhance your cardiovascular health or simply interested in understanding the science behind these vital nutrients, join us as we explore the role of vitamin D3 and K2 in promoting a healthier heart.
The Role of Vitamin D in the Body
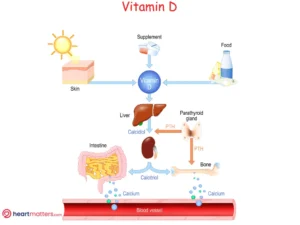
The body produces Vitamin D when the skin is exposed to sunlight. It can also be obtained through certain foods such as fatty fish, egg yolks, and fortified dairy products. The liver and kidneys convert vitamin D into an active form necessary for various bodily functions.
In addition to its role in bone health, vitamin D has been shown to have anti-inflammatory and immune-modulating properties. These properties may reduce the risk of cardiovascular disease.
Vitamin D and Cardiovascular Disease
Numerous studies have investigated the association between vitamin D status and cardiovascular disease. There are several potential mechanisms by which vitamin D may protect against cardiovascular disease. One possibility is that vitamin D may help reduce inflammation and oxidative stress, which are important risk factors for cardiovascular disease. Vitamin D may also help improve insulin sensitivity and regulate blood pressure, which are important for maintaining cardiovascular health.
Impact of a low Vitamin D level on Cardiovascular Disease
In 2019, a study published in BMC Cardiovascular Disorders analyzed data from 25 studies that included 10,099 cardiovascular disease (CVD) cases. The goal was to understand how vitamin D levels relate to the risk of CVD.
The study found that when vitamin D levels decrease, the risk of CVD (which includes heart-related conditions) increases. Specifically, it showed that a low serum vitamin D level raised the risk of CVD by 44%. This increase in risk applied to both the mortality rate of CVD (which increased by 54%) and the overall incidence rate of CVD (which increased by 18%).
Supplementing Vitamin D and Cardiovascular Disease
In a study published in JAMA Cardiology 2020, researchers analyzed data from 21 randomized clinical trials involving 83,291 patients. These patients were divided into two groups: one group received vitamin D supplements, and the other received a placebo. The average age of the participants was approximately 66 years, with a majority being female.
This study’s main focus was to understand whether vitamin D supplementation significantly impacted cardiovascular health. Surprisingly, the results showed that vitamin D supplements did not reduce major adverse cardiovascular events, including heart attacks and strokes. Additionally, the two groups had no significant difference in cardiovascular disease-related mortality or all-cause mortality.
These findings remained consistent regardless of gender, baseline vitamin D levels, the form of vitamin D used (daily or bolus dosing), or whether calcium supplements were taken concurrently. In summary, despite the hope that vitamin D supplementation might improve cardiovascular health, this study did not find any substantial benefits in preventing major heart-related events or mortality.
In light of the current data, it appears that maintaining a healthy vitamin D level within the normal range is advisable for cardiovascular health. If you have a deficiency, working to bring your levels into the normal range may offer benefits. However, once you’re within the normal range, there doesn’t seem to be added cardiovascular protection from elevating vitamin D levels beyond what is considered normal.
How to Get Enough Vitamin D
The recommended daily vitamin D intake varies depending on age, sex, and other factors. However, most people can obtain enough vitamin D through sun exposure and diet.
For optimal vitamin D intake, consider getting natural sun exposure through outdoor activities and incorporating vitamin D-rich foods into your diet, such as fatty fish, fortified dairy products, cereals, and UV-exposed mushrooms. It’s worth noting that exposing mushrooms to UV (from sunlight or in a laboratory) increases the amount of vitamin D in mushrooms by nearly eightfold. Reading nutrition labels can help you track your vitamin D intake from food sources. If you still have concerns about deficiency, supplements can be helpful. Alternatively, brief, direct skin exposure to sunlight for around 15 minutes a few times a week can also fulfill your vitamin D needs.
Supplements may be recommended for individuals who do not get enough vitamin D through sun exposure and diet. It is important to talk to a healthcare provider before taking any vitamin D supplements, as excessive amounts can be toxic.

Vitamin K2 and Vitamin D3
In addition to vitamin D, vitamin K2 may also help maintain cardiovascular health. Vitamin K2 activates proteins that help regulate calcium metabolism in the body. This is important because calcium buildup in the arteries can increase the risk of heart disease.
Vitamin K2, a lesser-known but equally important form of vitamin K, plays a crucial role in various physiological processes, with emerging research shedding light on its potential benefits for heart health. Unlike its counterpart, vitamin K1, which primarily supports blood clotting, K2 exhibits unique properties that contribute to bone metabolism, arterial health, and calcium regulation. Specifically, K2 activates proteins responsible for directing calcium to the appropriate locations in the body, such as the bones and teeth, while preventing its deposition in soft tissues like arteries. This function helps maintain bone density and reduces the risk of arterial calcification, a significant contributor to cardiovascular disease. Moreover, vitamin K2 has been associated with lower rates of arterial stiffness and improved endothelial function, further emphasizing its potential as a cardiovascular ally. As research continues to uncover the intricate mechanisms underlying its effects, incorporating vitamin K2-rich foods or supplements into one’s diet may offer valuable support for overall heart health.
Coronary artery Calcification
Coronary artery calcification (CAC) is the accumulation of calcium deposits in the coronary arteries. Its progression is a significant predictor of acute myocardial infarction (heart attack) and cardiovascular mortality. The presence and advancement of CAC can signal an increased risk of heart-related events. One way to measure CAC is with a CT calcium score.
In this context, vitamin K2 has drawn attention for its potential protective role in the progression of CAC. Vitamin K2 is believed to be vital in regulating calcium metabolism within the body. It helps direct calcium away from arterial walls and soft tissues, which can contribute to the development of arterial calcification. Instead, it facilitates deposition into bones and teeth, supporting their health.
What does the data say?
Combining vitamin K2 with vitamin D3 promotes a more balanced distribution of calcium within the body, potentially affecting systemic calcification and reducing arterial stiffness. Some propose this supplementation approach to enhance cardiovascular health and potentially improve outcomes.
Numerous clinical randomized trials are underway to explore the potential of Vitamin K2 in mitigating the progression of coronary artery calcification (CAC). For instance, an ongoing study is actively assessing the influence of Vitamin K2/D3 supplementation on coronary artery calcification. With the study’s methodology linked here, the eagerly awaited results may offer valuable insights into this realm of research.
Additionally, a study has delved into the potential effects of Vitamin K2 and D3 supplementing in individuals with aortic valve calcification. In the Danish AVADEC Trial, researchers embarked on a two-year supplementation regimen involving high-dose vitamin K2 (720µg/day) and vitamin D (25 µg/day). Intriguingly, this study did not yield a significant reduction in the progression of aortic valve calcification.
Research has shown that combining vitamin D3 and vitamin K2 supplements may be more effective in reducing cardiovascular disease risk than vitamin D3 supplementation alone. However, further research is required to validate the preliminary findings satisfactorily. Always check with your healthcare professional to determine which strategy may suit your particular circumstances.
Conclusion
In conclusion, the relationship between vitamin D and K is a topic of growing interest in the realm of cardiovascular health. While vitamin D plays a vital role in calcium absorption and bone health, vitamin K is emerging as a crucial regulator of calcium distribution within the body, potentially reducing the risk of calcification in arteries and valves.
Research has shown that the combined supplementation of vitamin D3 and vitamin K2 holds promise in supporting cardiovascular health. These supplements may influence systemic calcification and arterial stiffness, potentially leading to improved outcomes for individuals at risk of heart-related issues.
However, while these findings are encouraging, further research is necessary to fully understand their extent of benefits and precise role in cardiovascular health. Moreover, individual needs can vary, making it crucial to consult a healthcare provider before introducing vitamin supplements.